RITE (Residency In Training Exam)
What is RITE Exam:
- RITE is an exam held for all neurology residents PGY2-PGY4 in Feburary of every year.
Structure:
-
It consists of 425-Question test over 3.5 hours, divided as follows:
- Anatomy 10%
- Behavioral/Psych 10%
- Clinical adult 20%
- Clinical pediatrics 10%
- Contemporary issues 3%
- Neuroimaging 13%
- Pathology 10%
- Physiology 14%
-
Distributed over 3 sections:
- 1st and 3rd – Multiple choice
- 2nd – Imaging section
- Pathology
- CT, MRI
- EEG
- EMG
Aim:
- It is aimed to show you your areas of strength and weakness in order to prepare you for the board exam.
- For the program directors, it shows them if the program is successful in enriching the residents with the skills they need.
Importance for the Neurology board:
- The RITE scores correlate with the ABPN pass rate, as follows:
- 100% of those who scored in RITE > 75% (correct answers), passed the ABPN board on first attempt.
- 99% of those who scored in RITE > 65% (correct answers), passed the ABPN board on first attempt.
- Bottom line: if you score > 65% correct answers, you have high chances to pass the ABPN board exam.
Is the RITE exam similar to ABPN exam?
- NOT AT ALL
- RITE is more directed towards basic science, neurology board is directed to clinical science
- Very little of RITE questions will be encountered in board exam
- Neurology boards are WAY easier than RITE
- ABPN pass rate in 2020 was 91%
RITE Score Report:
- You will get report as "Percentile Rank" and "Percent Correct Answers"
- If you are in the 99 percentile per your class, this means you are one of the highest scoring 10 residents per your class (throughout US, 1000 neurology resident per PG year).

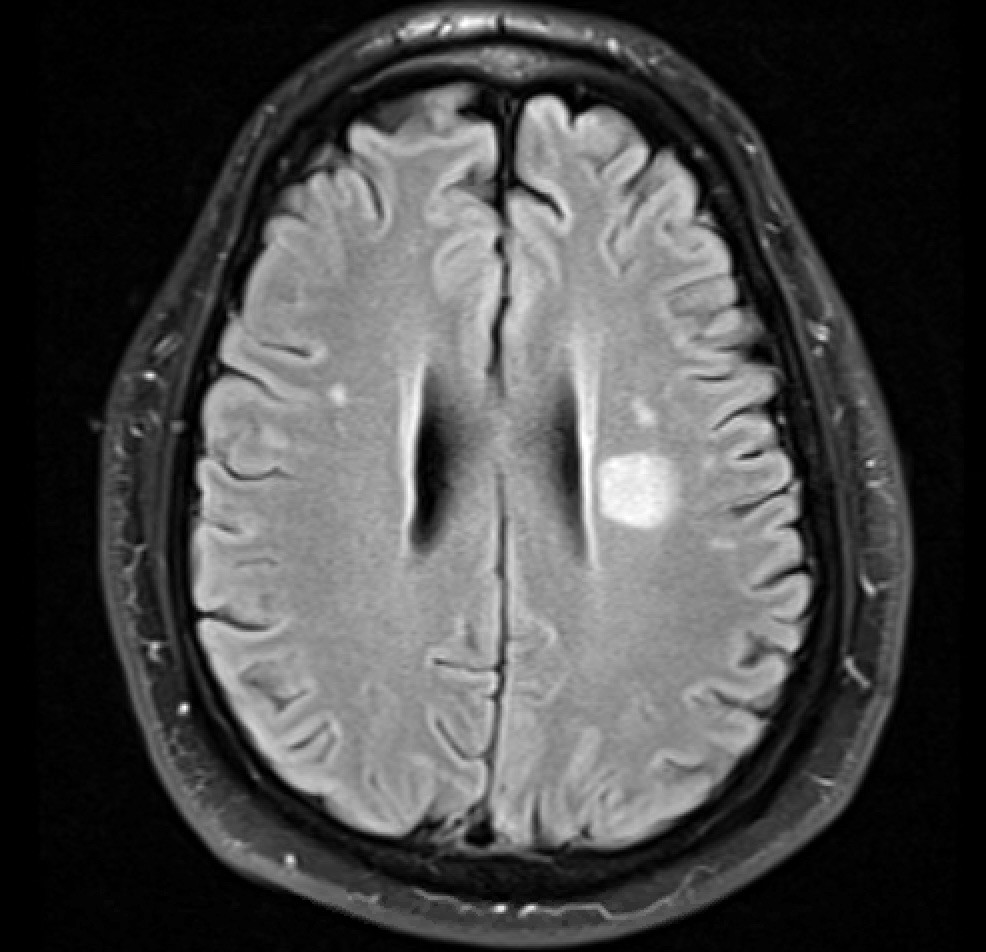
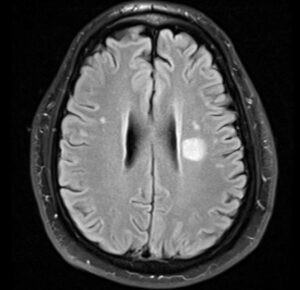
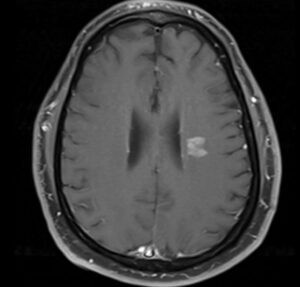

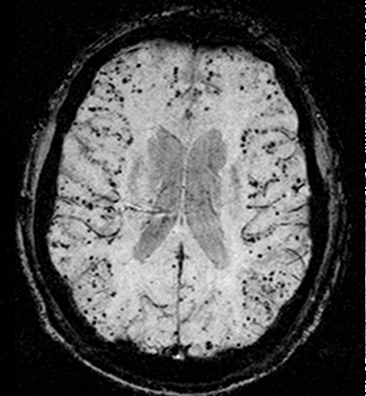
![Cerebral_amyloid_angiopathy_(CAA)-MRI By Marvin 101 [CC BY 3.0 (https://creativecommons.org/licenses/by/3.0)], from Wikimedia Commons](https://neurologyresidents.com/wp-content/uploads/bb-plugin/cache/Cerebral_amyloid_angiopathy_CAA-MRI-landscape-feda5685e321ed812b6f6b2e76ed4b88-5b82dfebd5f4b.png)





















